RFID and IoT deliver unbiased visibility throughout the entire supply chain. Where is the Value? Bayer Case Study
26:59
Learn how RFID delivers value in the pharmaceutical supply chain and get insight on our project carried out for Bayer S.p.A. in Italy.
Related Videos
In RFID Products
-
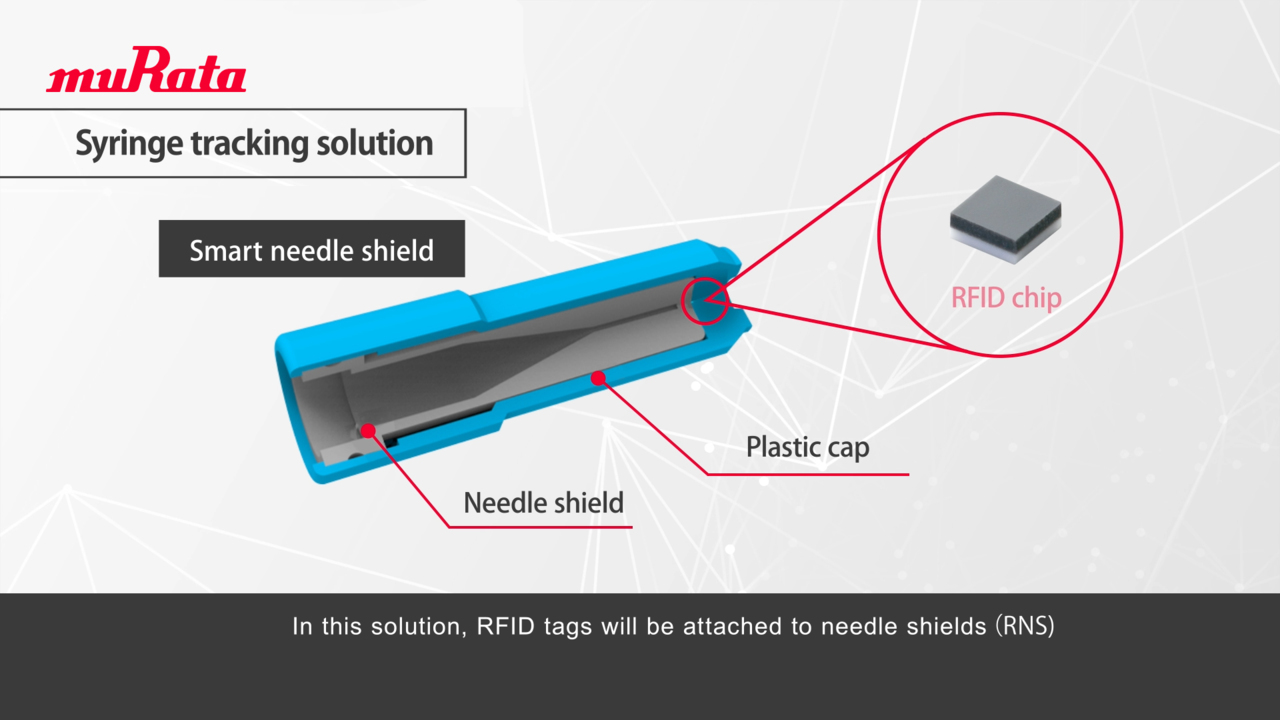
Play video Introducing Murata’s Syringe Tracking Solution
Introducing Murata’s Syringe Tracking Solution
FDA 21 CFR 610.14 requires chemical containers to be provided with information such as ingredients and manufacturing lot numbers for inspections using identity testing means and for error-free operations. Murata proposes syringe management solution
3:21
-
Play video Embedding RFID tags to tires and evaluation support
Embedding RFID tags to tires and evaluation support
To embed an RFID tag in a tire, it is necessary that the RFID tag be designed with consideration given to the rubber material and internal metals used for the tire and that enhanced communication characteristic evaluations be made on the RFID tag. Be
3:41
-
Play video A fully connected and transparent food supply chain with RFID
A fully connected and transparent food supply chain with RFID
In this RFID solution deployed by an important Italian food company, RFID labels are applied to all shipping units in order to accurately track the products in the inbound and outbound logistics processes.
1:47
-
Play video RFID technology helps optimise logistics processes and fight the grey market
RFID technology helps optimise logistics processes and fight the grey market
Murata ID Solutions’ RFID technology has helped Italian fashion brand Liu Jo strengthen protection against grey market while driving new efficiencies across its supply chain.
1:58
-
Play video Introduction to the id-Bridge™ Standard Service
Introduction to the id-Bridge™ Standard Service
We introduce you here to the id-Bridge™ Standard service, which contributes to improving logistics operations by using RFID.
3:09
-
Play video Introduction to Example of Implementation of id-Bridge Standard™ at Tokyo Logistics Center (Logistics Center Site Supervisor Version)
Introduction to Example of Implementation of id-Bridge Standard™ at Tokyo Logistics Center (Logistics Center Site Supervisor Version)
id-Bridge™ Standard has been implemented at Murata's Tokyo Logistics Center. We look here at what kind of system it is and what kind of effects have been obtained by using RFID in logistics.
5:25