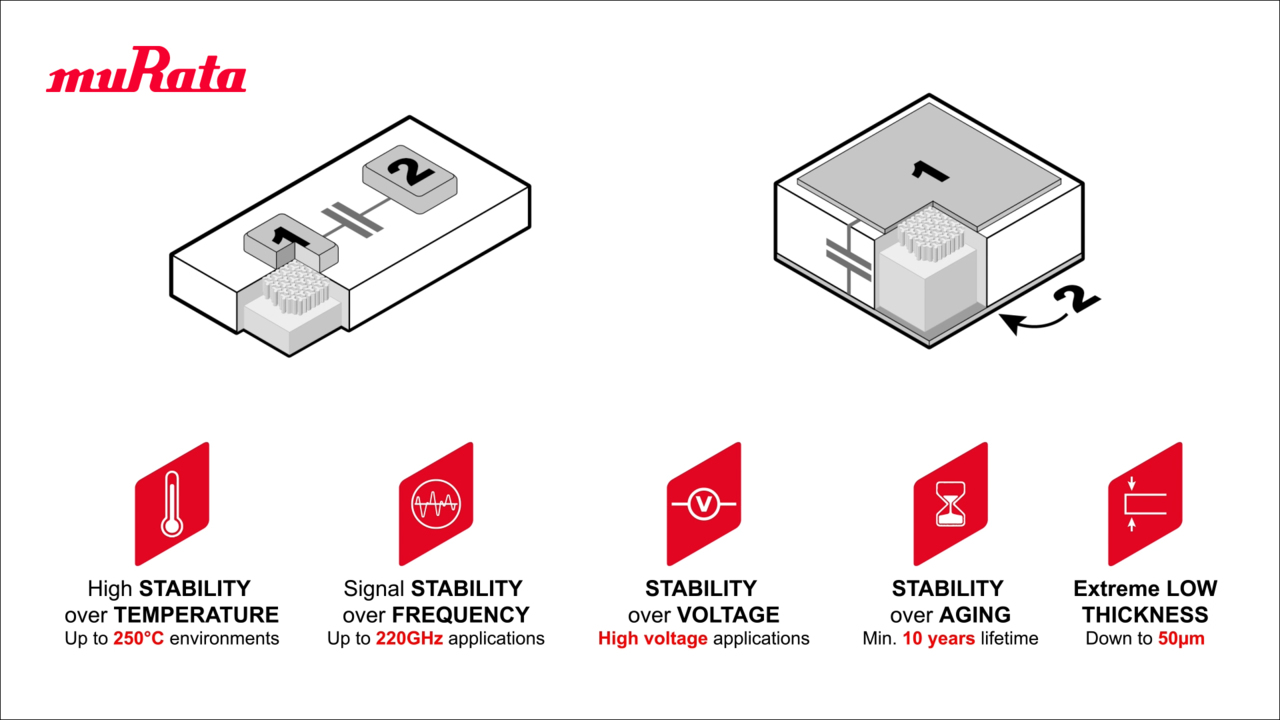
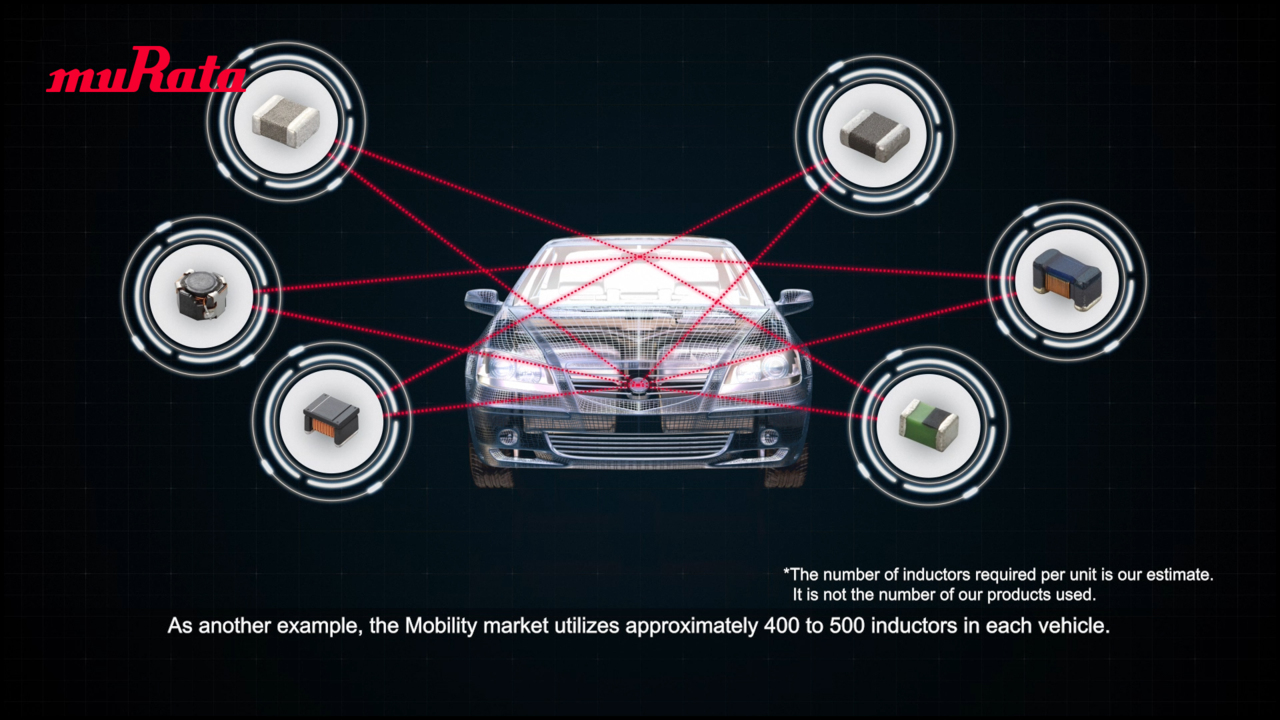
Introducing Murata’s Syringe Tracking Solution
3:21
Description
Related Videos
FDA 21 CFR 610.14 requires chemical containers to be provided with information such as ingredients and manufacturing lot numbers for inspections using identity testing means and for error-free operations. Murata proposes syringe management solution using RFID micro tags. The RFID solution saves you from having to do visual inspections. Information can be scanned after packing into sealed containers, which minimizes contamination risks as well as changes in manufacturing and design.
View More
View Less